I still remember the nervous walk into the clinic — hopeful, but wary of what a new treatment might mean for my daily life. Many people feel the same when they consider an implanted device to manage chronic pain.
This introduction will set clear expectations: what is typical after surgery, which issues are related to the implant, and when to seek help.
These systems work by altering signals in the spinal cord to reduce pain. Trials help most patients decide if the full implant is worth it.
Outcomes vary. Some patients sleep better, use fewer medications, and regain activity. Others need reprogramming or face rare complications. Knowing timelines and warning signs helps you stay safe and get the most from treatment.
Key Takeaways
- Expect normal surgical soreness but watch for device-related issues like lead movement.
- The system modifies cord signaling and may not “fix” the source of pain.
- Risks range from common, mild events to rare, serious problems with different timing.
- Most candidates first try a short trial to measure benefit before permanent implant.
- Early detection of infection (often within 2–8 weeks) can prevent removal.
- Newer stimulation modes can reduce uncomfortable tingling for some people.
Why spinal cord stimulation is used for chronic pain today
Many people turn to implanted electrical treatments after months of medication, physical therapy, and injections offer limited relief.
When conservative care fails, spinal cord stimulation becomes a second-line treatment. Doctors consider it for persistent conditions such as failed back surgery and complex regional pain. It also helps neuropathic and some visceral pain problems when other options fall short.
Where it fits in a broader plan
Stimulation is not a lone cure. It is added to a multimodal pain management plan that includes meds, exercise, therapy, and behavioral care.
- Patient selection uses imaging and psychological screening to improve outcomes.
- A temporary trial confirms meaningful benefit before permanent implant.
- Programming and waveform changes tailor therapy to the pain map and comfort.
- Active rehab and regular follow-up, including reprogramming, boost long-term success.
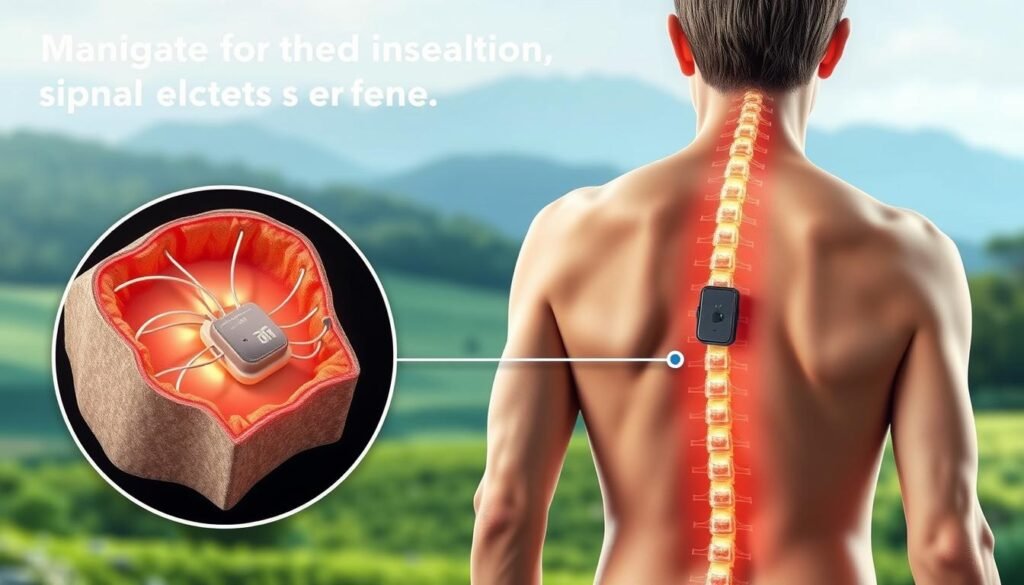
How a spinal cord stimulator works inside the body
A clear picture of the parts helps you understand what to expect after implantation.
Leads sit in the epidural space, the thin channel just outside the dura. Tiny electrodes rest next to the spinal cord protective layers to alter pain signaling. Placement level varies: thoracic leads often treat leg and back pain while cervical leads target arm symptoms.
The pulse generator is the system’s power hub. It sits under the skin near the buttock or abdomen and sends programmed impulses through the leads. Patients use a handheld remote to adjust settings within safe limits for activity and position.
Traditional therapy creates paresthesia — a tingling that replaces discomfort. Newer sub‑perception modes relieve pain without a felt sensation. Choices like high frequency, burst, or high‑density waveforms help balance comfort and coverage.
How signals change pain sensing
- Stimulation influences pathways such as the spinothalamic tract, altering how the brain reads pain.
- Programs can modulate nearby muscle groups and nerve signaling to reduce symptom intensity.
- Proper programming and occasional reprogramming fine‑tune coverage and reduce uncomfortable sensations.
| Component | Location | Purpose |
|---|---|---|
| Leads | Epidural space (adjacent to dura) | Deliver electrical pulses near the cord to change pain signals |
| Pulse generator | Under skin (buttock or abdomen) | Power source that controls waveform, amplitude, and timing |
| Handheld remote | User carried | Adjusts settings within prescribed ranges for comfort and activity |
Remember: these devices change how the body processes pain; they do not repair structural problems in the spine.
Types of spinal cord stimulators and what they mean for side effects
Not all implanted systems are the same; choices affect longevity, convenience, and risks.
Three main device classes exist: a conventional IPG with a fixed battery, a rechargeable IPG, and rare radiofrequency units that use external power. Conventional units suit lower power needs and often need surgical battery replacement every 3–5 years.
Rechargeable models typically last 10–15 years and support higher output. That higher output can give broader coverage for lower back and leg pain and may reduce the need for frequent reprogramming.
Battery, generator placement, and daily use
Generator pockets are usually at belt level in the abdomen or buttock. Placement trades off comfort, ease of charging, and how the pocket feels when you sleep.
- Conventional vs rechargeable: compare maintenance, longevity, and likely future surgery.
- Higher-output rechargeable devices often better cover tough pain patterns.
- Radiofrequency systems are uncommon today because implantable devices improved.
- Multi‑program presets let patients switch pulse profiles for sitting, standing, or walking.
| Type | Battery life | Notes |
|---|---|---|
| Conventional IPG | 3–5 years | Lower maintenance but needs surgical replacement |
| Rechargeable IPG | 10–15 years | Longer life, supports higher output and wider coverage |
| Radiofrequency | External power | Rarely used; less common with modern devices |
Choosing the right unit is individual. Your pain pattern, lifestyle, and willingness to recharge guide the best option. Good education on charging and remote use reduces avoidable interruptions in stimulation and lowers the chance of generator pocket discomfort.
Who is a candidate—and who may be at higher risk for side effects
A stepwise evaluation helps identify who will likely benefit and who may face higher risks.
Typical candidates are people with chronic pain that has not improved after medications, injections, physical therapy, or prior surgery. Imaging and a documented history of prior treatments are usually required by insurers before approval.
Psychological and medical risk factors
Psychological screening is common because conditions such as depression or anxiety can lower the chance of a good outcome. Managing mental health first often improves results.
High opioid use, active smoking, older age, and long pain duration are linked with reduced benefit and higher complication rates. These are often modifiable; quitting smoking and optimizing opioid doses can help.
Practical points before a trial
- Certain pain syndromes respond more predictably, which guides expectations for the trial.
- Insurers may require psychological clearance and detailed prior therapy records.
- Commitment to follow-ups, wound care, and possible reprogramming is essential.
Shared decision-making frames the process: the temporary trial serves as a real-world test of likely benefit and tolerability before permanent implantation, balancing risks against expected improvement in pain management and function.
Before the procedure: evaluations, anesthesia, and preparation
Preparing the body and the care team in advance makes the procedure smoother and safer.
Multidisciplinary checks help reduce risk. You will usually meet a neurologist, pain specialist, neurosurgeon, psychologist, and anesthesiologist. Each expert confirms your fit for the implant and flags issues that could change the plan.
What to tell your team
Disclose every medication, vitamin, and supplement. Blood thinners and some diabetes medicines often need adjustment. Only pause drugs under medical guidance.
Skin care, fasting, and tests
Pre-op bathing with antibacterial soap lowers infection risk at incision sites. Labs and imaging confirm readiness and guide anesthesia choices.
Fasting rules: no solid food for at least 8 hours and no clear liquids for at least 2 hours before general anesthesia. Your anesthesiologist will tailor plans based on health and history.
| Item | Why it matters | Patient action |
|---|---|---|
| Specialist visits | Confirm candidacy and address risks | Attend appointments and ask questions |
| Medication review | Prevents bleeding or blood sugar issues | List all meds; follow pause instructions |
| Pre-op hygiene | Reduces infection at the cord and pocket sites | Shower with provided soap the night before |
| Home prep | Supports day‑of discharge and recovery | Arrange ride, supplies, and help at home |
Bring device education materials and questions on the treatment day. Trial and permanent implants share most prep steps. Following instructions closely lowers complications and speeds recovery.
Trial vs. permanent implantation: where side effects can occur
A short, monitored trial run helps test whether the therapy truly eases daily pain.
How the trial works: Percutaneous leads are placed under fluoroscopic guidance, usually with light sedation. An external generator is attached to the skin or worn on a belt for a few days up to a week to mimic the full device.
Success is commonly defined as ≥50% pain reduction. If that threshold is met, the permanent implant procedure is often scheduled within 1–2 weeks.
Lead positioning and patient feedback
Fluoroscopy helps target the cord precisely. Patient feedback during placement refines electrode location to improve coverage for back or leg pain.
- Trials require keeping external parts dry—no bathing or swimming.
- Minor skin irritation, soreness, or bruising at insertion sites can occur.
- Paddle leads need a more invasive surgery and may require an overnight stay.
- An unsuccessful trial is reversible—leads are removed without damage to the cord.
“The trial gives real-world proof of benefit and tolerance before any permanent surgery.”
Common surgical side effects after implantation
Early recovery usually centers on wound comfort, restricted activity, and watching for expected bruising.
Incision pain, swelling, and bruising are common around both the back and generator pockets. Expect soreness that improves each day. Dressings are often removed in about three days and most incisions heal in 2–4 weeks.
Incision care and activity limits
Follow wound care instructions and keep the area clean and dry. Avoid stretching, twisting, or heavy reaching for roughly two weeks.
Broader limits on bending, lifting, and twisting usually last 1–2 months to protect lead position and prevent migration. Wear supportive clothing and avoid pressure on the generator pocket.
Anesthesia effects and day-of expectations
Anesthesia can cause short-lived nausea and grogginess. Many people go home the same day after recovery, while some stay 1–2 days for observation.
- Plan for a ride home and quiet rest the first 24 hours.
- Use ice and comfort measures per surgeon guidance; avoid placing direct pressure on the pocket.
- Turn the stimulation off when driving and follow instructions for resuming activities.
“Typical incisional discomfort improves steadily over the first couple of weeks; contact your care team if pain suddenly worsens or new symptoms appear.”
Device-related complications you should know
Device problems can change how well your therapy covers painful areas and may show up suddenly. These issues most often involve lead movement, electrode failure, or local pain at the generator pocket.
Lead migration and electrode failure
When leads shift in the epidural space, stimulation coverage can shrink or move. You might notice loss of relief or new areas of sensation.
Electrode failure shows as inconsistent coverage, sudden loss of paresthesia, or unexpected jolts. Report these changes right away.
Device damage, reprogramming needs, and generator pocket pain
Minor problems often respond to in‑office reprogramming. Technicians can adjust waveform, amplitude, or contact mapping to restore coverage without surgery.
If hardware is damaged by a fall or extreme twisting, revision surgery may be required to reposition or replace leads.
Generator pocket pain can cause pressure or tenderness. Adjusting placement, changing belt habits, or small surgical tweaks can reduce discomfort.
- Avoid trauma and intense twisting after surgery to protect leads and the cord.
- Anchoring sutures at permanent implantation lowers migration risk compared with trial leads.
- Schedule periodic checks to confirm device integrity and battery status.
- Report sudden jolts or shocks immediately—these can signal programming or hardware faults.
“Many coverage issues can be solved without further surgery through skilled reprogramming.”
Realistic expectation: occasional tweaks to the system are normal and part of successful long‑term treatment.
Infections: signs, timing, and when removal may be required
Wound infections often show up within weeks, and prompt action can save the device and recovery timeline.
Early warning signs include growing redness, swelling, warmth, or drainage at the incision or pocket. Fever or new worsening pain are also red flags. These signs most commonly appear between 2 and 8 days — and classically within 2–8 weeks after the procedure.
Rates, wound care, and risk reduction
Infection occurs in about 3.4%–10% of cases and is the most frequent reason for device removal. Fast action can sometimes save the system and avoid more invasive treatment.
- Care for incisions for the first 7–10 days: keep clean, change dressings as instructed, and avoid soaking wounds.
- Practice strict hand hygiene and do not submerge the area until your team clears you.
- Monitor temperature and report systemic symptoms like fever or chills promptly.
| Topic | What to watch for | Typical outcome |
|---|---|---|
| Early, superficial infection | Localized redness, mild drainage, low fever | Often responds to oral antibiotics and close follow-up |
| Deep or pocket infection | Spreading redness, increased pain, persistent drainage, higher fever | May require device removal and IV antibiotics |
| Risk factors | Smoking, uncontrolled diabetes, immunosuppression | Higher chance of infection and need for removal |
| Prevention | Pre-op skin prep, sterile technique, post-op wound care | Lower infection rates when guidelines followed |
“Early detection and coordinated care improve the chance of treating infection without permanent removal.”
What to expect if removal is needed: Removing hardware is safe and often followed by a course of antibiotics. Re-implantation may be considered once the body is clear of infection and healing is complete.
Neurological and dural complications: rare but serious
Although most recoveries are smooth, a small number of people develop nerve or dural complications that need prompt care.
Dural puncture happens when the needle or lead pierces the dura mater and allows cerebrospinal fluid (CSF) to leak. Loss of CSF changes pressure around the brain and can cause a severe, positional headache.
Classic signs include a headache that worsens when upright, neck stiffness, nausea, and light sensitivity. If these appear after the procedure, contact your team promptly; many patients improve with conservative care or a targeted blood patch.
Dural leak treatment and recovery
Initial care often includes bed rest, fluids, and pain control. A blood patch can seal the leak when conservative steps fail. Rarely, more invasive care is needed for persistent symptoms.
Nerve injury, cord trauma, and hematoma
Direct injury to a nerve or the cord is uncommon but can cause new weakness, loss of sensation, or bowel/bladder changes. An epidural hematoma—bleeding in the epidural space—can produce rapid decline and is a surgical emergency.
- Report immediately any new weakness, numbness, or trouble walking.
- Early imaging and exam speed diagnosis and treatment.
- Prevention relies on experienced operators and careful fluoroscopic technique.
Most procedures are uneventful. Still, follow activity limits to reduce mechanical stress on the epidural space and track new neurologic symptoms closely. If you notice worrying changes, seek same‑day medical attention.
Learn more about conditions commonly treated with this therapy at common candidate conditions.
spinal stimulator side effects: how often they happen and how severe
Clear numbers help people weigh risks and benefits. Reported data indicate about 30%–40% of patients experience one or more complications after implantation. Many of these are minor and fixable.
Common problems include lead migration and electrode failure. These often cause loss or change of coverage and usually respond to in‑office reprogramming or a minor revision.
Understanding complication rates and real-world expectations
Infection rates range from 3.4% to 10%, most often in the first few weeks. Early recognition and antibiotics can often prevent device removal.
- Minor or correctable: most events are handled with programming, antibiotics, or small procedures.
- Most serious events: neurologic injury or hematoma is rare but requires urgent care.
- Benefits: high-frequency approaches report average pain relief of 54%–87% in studies, and over 60% of patients reduce opioid use.
| Category | Reported rate | Usual outcome |
|---|---|---|
| Any complication | 30%–40% | Often minor; managed without major surgery |
| Infection | 3.4%–10% | Oral/IV antibiotics or removal if deep |
| Serious neurologic events | <1% (rare) | Urgent imaging and possible surgery |
“Ask your provider for center-specific rates and typical outcomes before choosing treatment.”
Bottom line: individual results vary by condition, device type, and follow-up care. Open communication with your team can catch minor problems early and keep most people benefiting from improved function and reduced medication needs.
Managing and minimizing side effects over time
Good long-term management depends on simple habits and timely follow-up care.
Early activity limits matter. Avoid bending, twisting, and heavy lifting for 1–2 months after the procedure. These precautions lower the risk of lead movement and preserve early pain relief.
Schedule follow-ups at about 10–14 days for wound checks and suture removal. Regular visits let clinicians reprogram the device to refine coverage and reduce uncomfortable paresthesia.
Practical steps for better long-term management
- Keep a brief symptom and activity diary to guide programming tweaks.
- Practice gentle posture habits; avoid pressure on the generator pocket.
- Follow charging routines and check battery status to prevent interruptions.
- Expect seasonal or activity-based setting adjustments as your routine changes.
When to call your care team
Red flags: fever, growing redness, increasing drainage, severe positional headache, sudden loss of coverage, new weakness, numbness, or jolts.
| Concern | What to do | Usual outcome |
|---|---|---|
| Worsening wound | Contact clinic same day | Antibiotics or close monitoring |
| Sudden loss of coverage | Call for reprogramming or assessment | Often fixed with in‑office adjustments |
| Severe headache after surgery | Seek immediate care (possible CSF leak) | Bed rest, blood patch, or targeted treatment |
| New weakness or numbness | Emergency evaluation | Imaging and prompt treatment if needed |
“Early reporting and proactive reprogramming often convert minor problems into quick wins.”
Team approach: pain specialists, therapists, and mental health support improve long‑term outcomes. Stay engaged and report changes promptly to protect benefit and comfort.
Living safely with a spinal cord stimulator day to day
Living with an implanted device means learning a few practical rules to keep therapy reliable.
Driving, swimming, airport security, and metal detectors
Turn the system off before driving or operating heavy equipment. Sensation shifts can distract you and change how you react behind the wheel.
Swimming is safe after a permanent implant once the incision has fully healed. During the external trial, keep leads dry and avoid pools, tubs, or open water.
Airport scanners and metal detectors will detect an implanted generator. Carry the device ID card, inform security, and switch the unit off before passing through to avoid harmless interference.
Imaging rules: X-ray, CT, and MRI compatibility
X-rays and CT scans are typically safe when the device is powered off. Always tell imaging staff about your implant so they can plan appropriately.
MRI safety depends on the model. Some units are conditionally compatible; others are not. A specialist must verify your device model and any scan-specific limits before scheduling an MRI.
- Keep documentation of device model and MRI conditionality in your wallet or phone.
- Store chargers and remotes where you can access them while traveling.
- Reduce pocket irritation by changing belt position, using soft padding, and adjusting exercise routines.
- Most people return to desk or light duties within 1–2 weeks with surgeon approval.
“Everyday safety steps are simple and protect both your comfort and long-term benefit.”
Plan ahead: pack charging gear, carry clinic contact info, and follow wound‑healing timelines. These small habits keep treatment steady and let you focus on daily life with less pain.
Effectiveness, pain relief, and quality-of-life trade-offs
Real-world gains often show up as better sleep and more movement, not just lower numbers.
High-frequency studies report average pain reduction between 54% and 87%. Many people achieve meaningful relief and return to activities that mattered before pain limited them.
Pain reduction ranges and opioid-sparing benefits
Over 60% of patients reduce or stop opioid medications after a successful course of care. Discuss tapering plans with prescribers before changing doses.
- Set realistic targets: moderate to high percent reductions are common, but not guaranteed for everyone.
- Quality of life: improvements in sleep, function, and mood often count more than raw scores.
- Durability: benefits can change over years and may need reprogramming or hardware updates.
- Personal goals: define goals like walking distance or hobby return, not just pain numbers.
“Outcomes depend on diagnosis, device selection, and active rehab and follow-up.”
For balanced guidance and pooled data, see recent reviews that summarize outcomes for persistent back pain after failed lower back surgery.
Alternatives, costs, and insurance considerations in the United States
If a trial doesn’t bring lasting relief, there are practical paths to keep pursuing better pain control.
Non‑implant options include medication optimization, focused physical therapy, targeted injections, and radiofrequency ablation. Behavioral therapies and multidisciplinary pain management programs help many people improve function without further surgery.
When devices or the trial fall short
Other neuromodulation choices—different generators, paddle leads, or peripheral nerve systems—may be offered if one device does not meet goals. Reprogramming or minor revisions often restore benefit.
Insurance and cost in the U.S. usually require documentation of prior conservative care and successful trial results before approving a permanent implant. Psychological screening is often part of authorization. Out‑of‑pocket costs vary by device type, facility fees, and future replacement or revision surgeries.
| Item | Typical range | Notes |
|---|---|---|
| Device & implant | $20,000–$50,000 | Depends on model and hospital fees |
| Revision or replacement | $5,000–$30,000 | Cost varies with complexity |
| Non‑implant treatments | $200–$8,000 | PT, injections, ablation costs vary |
| Insurance requirements | Documentation + psych eval | Keep detailed records to speed approvals |
Practical tips: verify MRI compatibility and coverage details with your insurer before any procedure. If problems persist, discuss reprogramming, revision, or safe explant. Keep thorough treatment records and ask your clinician for appeal letters if coverage is denied. Shared decision‑making keeps safety, cost transparency, and patient goals at the center of care.
Conclusion
A thoughtful plan that pairs technology, rehab, and lifestyle change often delivers lasting improvements in pain and function.
For selected people, a trial of spinal cord stimulation predicts whether long-term treatment will bring real relief and better daily activity. Benefits commonly include reduced medication needs and improved quality of life, while problems range from routine wound soreness to rare neurologic events.
Stay engaged: keep regular follow-up, expect occasional reprogramming, and follow wound and activity guidance. Power the device off before driving and confirm imaging or travel rules in advance. Report early signs of infection or new neurologic symptoms right away.
Talk openly with a multidisciplinary team about goals, risks, and costs. With careful selection and partnership, many people reach meaningful pain relief and regain valued activities.

