Living with persistent pain can feel like a quiet ache in every choice you make. I know how tiring small tasks become and how hope can feel fragile. This guide meets you there — practical, clear, and kind.
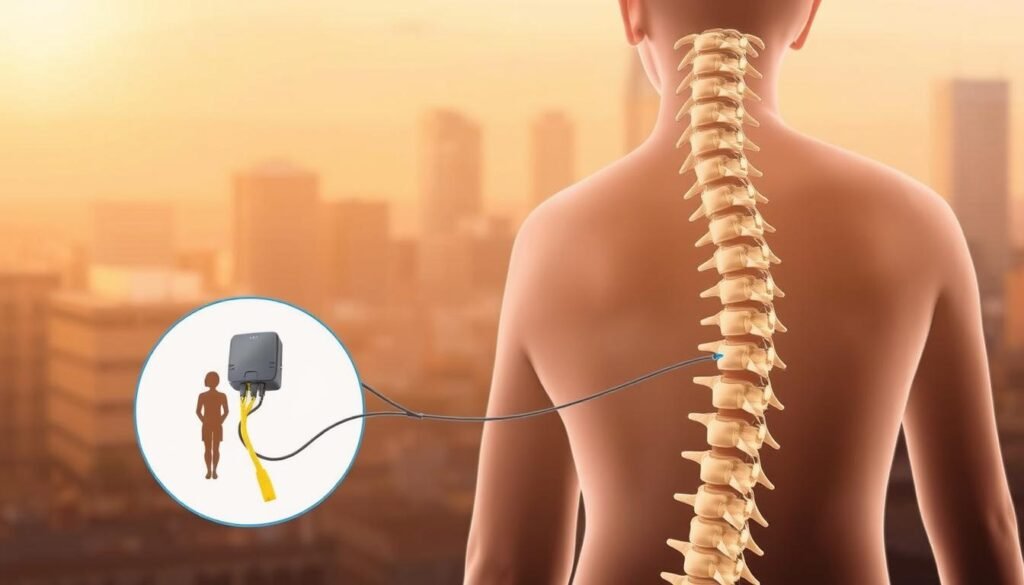
Boston Scientific offers a modern path to managing chronic pain through an extensive SCS portfolio built for daily life. Systems like WaveWriter Alpha deliver options such as FAST Therapy for paresthesia-free relief and can target multiple areas at once.
The devices are designed for 24-hour use, let you turn stimulation on or off, and let you adjust settings with a secure remote. Success during a trial is commonly defined as at least a 50% reduction in pain, a simple milestone that helps patients and doctors decide next steps.
Safety note: do not charge the system while sleeping and follow your clinician’s guidance. For support, Patient Services is available at (866) 360-4747, 6 am–5 pm PT, Monday–Friday.
Key Takeaways
- These systems offer personalized pain therapy that adapts to daily needs.
- Trial success is typically defined as a ≥50% pain reduction.
- Devices allow 24-hour use, multi-area targeting, and easy remote control.
- FAST Therapy can provide paresthesia-free relief quickly with lasting outcomes.
- Contact Patient Services at (866) 360-4747 for help during the process.
What Is Spinal Cord Stimulation and How Can It Help with Chronic Pain?
Mild electrical pulses delivered near the spinal cord can change pain signals before they reach your brain.
Spinal cord stimulation is a therapy that uses carefully controlled pulses to interrupt pain messages along the cord so you feel less pain. It does not cure the underlying condition, but it can manage persistent pain from issues like failed back surgery and herniated disc problems.
Many people find SCS reduces their need for pain medication. Others use it alongside current medicines under a doctor’s guidance. A trial with temporary leads helps determine if you get meaningful pain relief—success is often defined as about a 50% drop in pain during that short test period.
Settings are personalized to where you hurt most, such as the low back or legs, and you can adjust levels at home with a secure remote. Options include paresthesia‑free programs and traditional paresthesia‑based approaches so your clinician can match the therapy to your comfort.
- Trial evaluates real‑world benefit before implant
- Personalized targeting for back and leg pain
- May reduce medication for some patients
Stay in close contact with your care team about pain associated with daily tasks. They will guide when to use stimulation and when to pause it for safety.
| Feature | What it does | Who it helps | When to try |
|---|---|---|---|
| Trial period | Temporary leads test relief | People unsure about benefit | Before permanent implant |
| Personalized settings | Targets low back or legs | Varied pain syndromes | During programming visits |
| Paresthesia options | Paresthesia‑free or traditional | Comfort‑based choice | Decided with clinician |
| Medication impact | May reduce opioid need | Those seeking lower meds | Monitored by physician |
Learn more about the specific conditions SCS can treat to see if this treatment might fit your care plan.
How the System Works: Stimulation, Leads, and Targeted Pain Relief
Understanding the basics makes choices easier. Small, programmable pulses travel from an implanted generator through thin leads to alter pain signaling near the spine. This setup is built so clinicians can aim therapy where you need relief most.
From electrical pulses to pain relief: an easy-to-understand overview
The implantable pulse generator sends precise electrical pulses along leads to change how pain signals reach the brain. You can switch between programs at home to match activities, positions, or daily needs.
Leads placement and programming to target back and leg pain
The Infinion Pro percutaneous lead can span up to three vertebral levels, helping treat multiple regions and reducing loss of benefit from small lead shifts.
https://www.youtube.com/watch?v=ot_gIGZdUWw
- Programming directs energy to specific contacts for focused relief.
- Multiple independent current control enables fine‑tuned neural dosing.
- Some settings feel like a light tingling; paresthesia‑free options aim for sensationless relief.
Hardware is made for durable contact and consistent therapy. Your care team will adjust settings over time and give practical guidance for sleeping and movement during recovery to protect lead position.
WaveWriter Alpha™ and Alpha™ Prime: Features Built for Personalized Care
WaveWriter Alpha™ and Alpha™ Prime deliver a portfolio built around precise targeting and easy daily control.
FAST Therapy is an automated, paresthesia‑free approach that can produce meaningful pain relief in minutes. Clinicians can confirm a response quickly, and real‑world evidence shows improvements in pain and function sustained to two years.
Precision tools include Multiple Independent Current Control (MICC) at 1% increments and Prism Targeting. These let clinicians “dose” stimulation to match your anatomy and symptoms.
Combination therapy options allow pairing waveforms for complex pain patterns. The Infinion Pro lead design supports multi‑area coverage and helps reduce loss of benefit from lead movement.
mySCS GO Therapy Controller gives a discreet, secure way to adjust programs at home without hassle. The portfolio also offers rechargeable and non‑rechargeable options to fit different lifestyles.
Some devices in the family support full‑body MRI access when all ImageReady MRI Full Body conditions are met, preserving diagnostic choices.
boston scientific spinal cord stimulator: Indications, Contraindications, and Who May Benefit
Knowing indications and contraindications helps people and clinicians pick the right candidates for therapy.
Indications: These cord stimulator systems are indicated as an aid in the management of chronic intractable pain of the trunk and/or limbs. Common uses include pain associated with failed back surgery syndrome, Complex Regional Pain Syndrome (CRPS) I and II, and intractable low back pain and leg pain.
Associated causes can include radicular pain syndrome, radiculopathies, epidural fibrosis, degenerative disc disease such as herniated disc pain that did not respond to prior care, arachnoiditis, and multiple back surgeries.
- People with chronic pain of the trunk or limbs who have not benefited from conservative care may benefit from scs therapy.
- For painful diabetic peripheral neuropathy of the lower extremities, the device is approved only with paresthesia‑based therapy; sub‑perception options like FAST are not FDA‑approved for DPN.
- A successful trial demonstrating meaningful pain relief is required before considering permanent implant.
Contraindications: Do not proceed with implantation for patients who cannot operate the system, who had a failed trial, who are poor surgical candidates, or who are pregnant.
Talk candidly with your physician about medical history and goals. SCS is an aid to management chronic pain and works best when matched to diagnosis, comfort, and approved labeling.
ImageReady™ MRI Access: Head-Only and Full-Body MRI Conditional Systems
Imaging matters when you live with chronic pain. Some implanted devices allow MRI exams, but only when every rule in the relevant ImageReady™ guideline is met for that exact model. Follow your clinician’s instructions to keep scans safe.
Full‑body MRI with WaveWriter Alpha and Alpha Prime under specific conditions
WaveWriter Alpha™ and WaveWriter Alpha™ Prime SCS systems with ImageReady Full Body Technology can permit full‑body MRI—but only under strict, model-specific conditions defined in the official guidelines. Your radiology team and physician must confirm the system model and follow the checklist precisely.
When MRI is not permitted and why conditionality matters
MR Conditional means a scan is allowed only if every listed condition is met. Systems without ImageReady technology must not be scanned. Risks include heating, lead movement, and device damage that can affect safety and long‑term performance.
“Always carry your device ID and tell the imaging center about your implant before scheduling an MRI.”
- Your care team will verify which guideline applies to your system.
- If MRI is not permitted, alternatives such as CT or ultrasound may be considered.
- Contact Patient Services or your clinician for help identifying the correct ImageReady MRI guideline.
Your Care Path: Trial, Implant Procedure, and Recovery
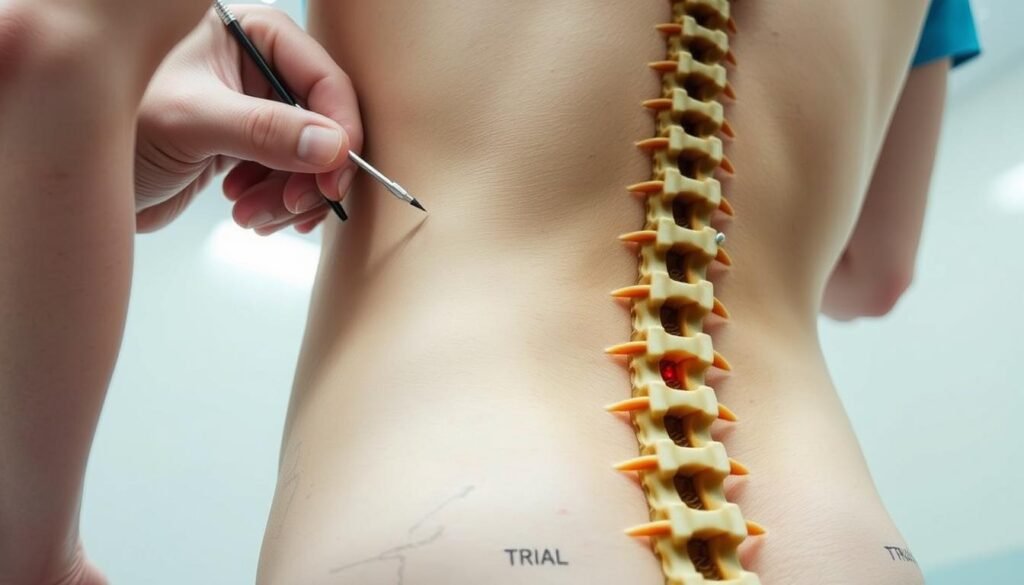
Begin with an outpatient test phase so you can judge relief during normal routines before any permanent procedure.
The trial experience
Temporary leads are placed in an outpatient setting and hooked to an external device. You wear the setup during everyday tasks to see if the therapy reduces discomfort.
Success is commonly defined as at least a 50% reduction in pain during the trial, a simple benchmark that helps patients and clinicians decide next steps.
Implant day and early recovery
If the trial is successful, a small pulse generator is implanted under the skin and connected to permanent leads during a short procedure. Your surgical team programs initial settings before you leave.
Limit activity for several weeks, follow incision care, and avoid motions your doctor warns against so leads can settle. Most people return to light activities first, then increase as cleared by their clinician.
| Step | What to expect | Patient action |
|---|---|---|
| Outpatient trial | Temporary leads, real‑world testing | Record pain and activities |
| Implant procedure | Pulse generator placed under skin | Follow pre/post‑op instructions |
| Recovery & follow‑up | Program adjustments, activity limits | Attend scheduled visits |
Do not charge the system while sleeping. Keep a simple pain journal to guide programming changes. For more detailed device guidance, see the SCS handbook.
Results You Can Measure: Clinical Outcomes and Real-World Evidence
Recent registries and trials report clear improvements in pain scores that last into the second year after implant.
Pain score and disability improvements sustained out to two years
Real-world data show FAST Therapy users achieved notable pain relief, with an average 5.6‑point NRS reduction and a 29‑point drop in ODI that lasted to two years.
These gains map to better daily function and often less disruption from pain during work and sleep.
FAST Therapy mechanism: surround inhibition with precise targeting and dosing
FAST is designed to engage surround inhibition, a targeted modulation approach that can act quickly.
Precise targeting and neural dosing rely on proprietary algorithms and Multiple Independent Current Control to deliver the right stimulation dose.
DPN data and registry insights: where paresthesia-based therapy applies
The RELIEF registry sub‑analysis reported an 81% responder rate and 95% sustained improvement at two years, reinforcing durability in real practice.
Label note: for diabetic peripheral neuropathy of the lower extremities, boston scientific systems are approved only with paresthesia‑based therapy; sub‑perception FAST is not FDA‑approved for that indication.
- Physicians and patients often value relief seen in minutes, enabling same‑visit confirmation and faster decisions.
- Outcomes vary by history and trial response; follow‑up fine‑tunes therapy for long‑term benefit.
Safety, Warnings, and Everyday Use
Knowing when to pause therapy keeps you safe during driving, certain procedures, and high‑risk tasks.
Using therapy safely: driving, machinery, and when to turn stimulation off
If you use paresthesia‑based programs, turn stimulation off before driving or operating machinery. Sensations can distract you and affect reaction time.
Sub‑perception stimulation lowers the chance of distraction, but always follow your clinician’s guidance about when to pause therapy.
Electromagnetic fields, charging guidance, and implanted device interactions
Patients without imageready mri compatibility must not undergo MRI — exposure can cause heating, lead movement, jolting sensations, or device damage.
Strong electromagnetic fields, such as theft detectors or industrial generators, may turn your stimulator systems off or cause uncomfortable stimulation. Move through gates and avoid lingering near large generators.
Never charge the system while sleeping. Build charging into daytime routines and keep the charger and controller in good condition.
| Risk | Why it matters | What to do |
|---|---|---|
| MRI (non‑ImageReady) | Heating, lead movement, device damage | Do not undergo MRI; discuss alternatives |
| High EMF exposure | Device switch‑off or uncomfortable pulses | Avoid lingering; inform staff of implant |
| Driving with paresthesia | Distracted driving risk | Turn stimulation off first |
| Diathermy | Serious thermal injury risk | Contraindicated—do not use |
| Other implants | Interference with pacemakers/ICDs | Tell clinicians and coordinate care |
- Carry your device ID card and show it before imaging or procedures.
- Contact Patient Services or your clinician for unusual sensations, alerts, charging issues, pregnancy, infection signs, or sudden pain changes.
- Simple habits and open communication with your care team make everyday life manageable with this system.
Conclusion
Modern implantable therapy offers a personalized route to lasting pain relief and improved daily function.
Spinal cord stimulator therapy delivers a customizable option for pain management that many people use to reduce discomfort from low back pain, failed back surgery, and herniated disc‑related pain.
The portfolio, including WaveWriter Alpha™, supports precise targeting, easy at‑home control, and ImageReady MRI access on eligible devices when all conditions are met. Candidacy depends on your history and a successful trial so you move forward with confidence based on real results.
Talk with your care team to tailor settings, follow safety habits (turn off paresthesia programs before driving; do not charge while sleeping), and plan next steps. Patient Services can help at (866) 360-4747, 6 am–5 pm PT, Monday–Friday.
You can pursue more comfortable days with the right system, guidance, and follow‑up.

